The CRISPR/Cas9 system has revolutionized genetic research as a precise, programmable genome-editing tool. Its accessibility and efficiency have empowered scientists to explore the genetic underpinnings of disease and develop therapies for conditions previously untreatable by conventional methods. By correcting patient-specific mutations, CRISPR/Cas9 enables personalized treatments for monogenic disorders, cancer, and chronic diseases. This article reviews the mechanisms, recent advancements, therapeutic applications, and ethical considerations of CRISPR/Cas9 in modern precision medicine.
Introduction to Genome Editing
Genome editing allows the precise modification of DNA at the level of individual bases. This technology has transformed biological research and holds immense potential for treating human genetic disorders. An ideal genome-editing tool efficiently alters specific sequences with minimal off-target effects. Early approaches relied on homologous recombination in yeast and mammalian cells, enabling researchers to study disease pathways in model organisms like mice. These breakthroughs paved the way for the development of targeted nucleases, including meganucleases, zinc-finger nucleases (ZFNs), and TALENs, which create site-specific double-strand breaks (DSBs) to trigger DNA repair mechanisms such as homology-directed repair (HDR) or non-homologous end joining (NHEJ).
HDR enables precise genetic corrections using a donor DNA template, while NHEJ often introduces insertions or deletions (indels), useful for gene knockouts but prone to off-target mutations. The advent of CRISPR/Cas9 significantly improved editing precision, allowing researchers to minimize unintended consequences while effectively correcting disease-causing mutations.
The CRISPR/Cas9 System
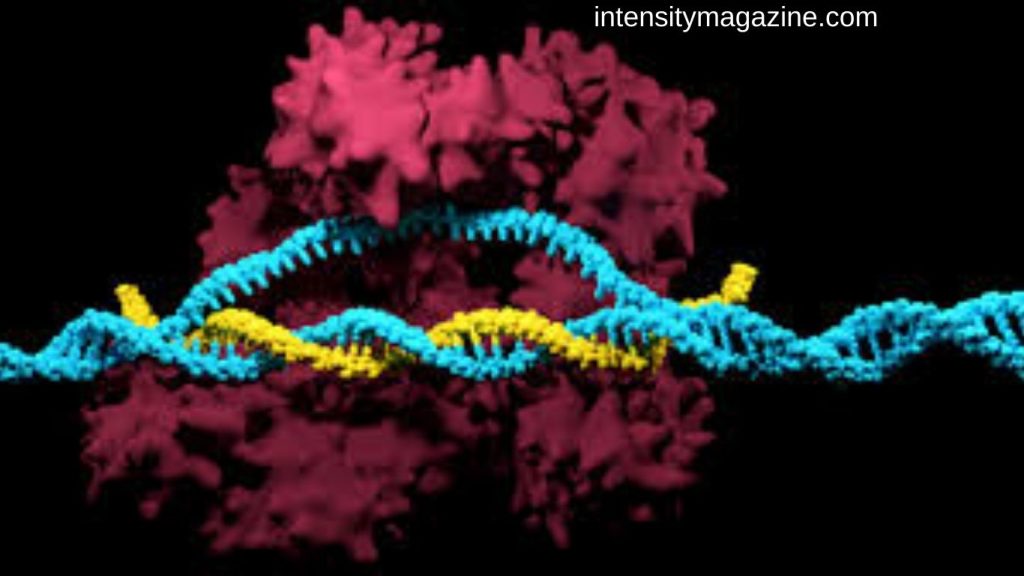
Discovered in bacteria as a viral defense mechanism, CRISPR/Cas9 employs a guide RNA (gRNA) to direct the Cas9 endonuclease to specific DNA sequences. Cas9 introduces DSBs near protospacer-adjacent motifs (PAMs), activating the cell’s natural repair processes. The gRNA consists of a CRISPR RNA (crRNA) complementary to the target sequence and a trans-activating crRNA (tracrRNA) that binds Cas9, forming a complex capable of precise DNA cleavage. This simple yet versatile system has become a standard in genome editing due to its efficiency, affordability, and ability to target multiple genomic regions simultaneously.
Mechanism of Action
CRISPR/Cas9 functions through two main DNA repair pathways:
Non-Homologous End Joining (NHEJ): Predominant in non-dividing cells, NHEJ rapidly joins broken DNA ends, often creating indels that disrupt gene function. This method is ideal for gene knockouts.
Homology-Directed Repair (HDR): HDR uses a homologous template, either endogenous or supplied externally, to enable precise genome modifications such as point mutation corrections or gene insertions. HDR efficiency increases during S and G2 phases of the cell cycle.
Recent Technological Advancements
Enhancing Precision
High-fidelity Cas9 variants, such as SpCas9-HF1 and eSpCas9, have been engineered to minimize off-target effects by improving DNA recognition. Base editing and prime editing now allow single-base conversions without creating DSBs, reducing risks associated with traditional CRISPR techniques. Prime editing employs a pegRNA and reverse transcriptase to insert, delete, or substitute nucleotides with high accuracy.
Advanced Delivery Methods
Efficient delivery of CRISPR components is critical for therapy. Lipid nanoparticles (LNPs) protect RNA components for cellular uptake, while viral vectors like adeno-associated viruses (AAVs) target specific tissues, such as the liver or lungs. Innovations in tissue-specific targeting use ligands or antibodies to deliver CRISPR precisely to intended cells, enhancing both safety and efficacy.
Applications in Precision Medicine
Genetic Disorders
Cystic Fibrosis (CF): CRISPR-Cas9 repairs CFTR gene mutations, restoring function in patient-derived lung cells and offering potential cures.
Sickle Cell Anemia: Editing the HBB gene or inducing fetal hemoglobin production corrects defective hemoglobin, producing healthy red blood cells.
Duchenne Muscular Dystrophy (DMD): Mutated exons in the DMD gene can be excised to restore the reading frame, enabling functional dystrophin production.
Cardiovascular Diseases: CRISPR enables the development of gene-based therapeutics and innovative research tools to understand disease mechanisms.
Cancer Therapy
CRISPR accelerates cancer research by creating targeted mutations in tumor suppressors and oncogenes. CAR-T cell therapy benefits from CRISPR by modifying T cells to enhance tumor recognition, persistence, and safety. Clinical trials show CRISPR-engineered CAR-T cells effectively target PD-1 and immune rejection genes, offering scalable and precise cancer therapies.
Viral Infections
CRISPR shows promise against chronic viral infections. In HIV, CRISPR excises integrated viral DNA, disrupting replication. In HBV, targeting covalently closed circular DNA (cccDNA) interrupts viral production. These strategies significantly reduce viral load and improve immune responses in preclinical models.
Rare Diseases and Personalized Medicine
CRISPR enables tailored therapies for rare or undiagnosed conditions. Successful interventions include correcting CEP290 mutations in Leber congenital amaurosis and upregulating SMN2 in spinal muscular atrophy models. Multiplexed genome editing allows simultaneous targeting of multiple genes, enhancing our understanding of complex gene networks. RNA editing techniques like REPAIR and RESCUE offer reversible, DNA-independent corrections for point mutations or splicing errors, expanding therapeutic potential.
Ethical, Legal, and Social Considerations
Germline editing raises ethical concerns due to heritable changes and the potential for unintended mutations. Designer babies, social inequality, and off-target effects pose significant challenges. Global regulatory frameworks vary widely, with countries like China adopting permissive policies while Western nations enforce stricter controls. Public perception is influenced by media, education, and cultural beliefs, emphasizing the need for transparent communication and responsible governance.
Future Perspectives
CRISPR/Cas9 is poised to transform precision medicine by enabling highly personalized treatments tailored to individual genetic profiles. Ongoing innovations in delivery methods, high-fidelity variants, and safety monitoring will broaden clinical applications. Ethical frameworks and global cooperation will be crucial for responsible integration into routine medical practice. The technology’s potential ranges from gene therapy and cancer treatment to antiviral strategies and rare disease management, offering hope for safer, more effective, and customizable healthcare solutions.
Frequently Asked Questions:
What is CRISPR/Cas9 and how does it work?
CRISPR/Cas9 is a genome-editing tool that uses a guide RNA (gRNA) to direct the Cas9 enzyme to specific DNA sequences. Cas9 creates a precise break in the DNA, which the cell repairs naturally, allowing targeted gene modifications.
How is CRISPR/Cas9 transforming precision medicine?
CRISPR/Cas9 enables personalized treatment by correcting patient-specific genetic mutations. It allows therapies for previously untreatable genetic disorders, cancers, and rare diseases.
What are the recent advancements in CRISPR technology?
Key innovations include high-fidelity Cas9 variants that reduce off-target effects, base and prime editing for precise single-base changes, and improved delivery methods using lipid nanoparticles and viral vectors.
Which diseases can be treated with CRISPR/Cas9?
CRISPR/Cas9 has shown promise in treating monogenic disorders like cystic fibrosis, sickle cell anemia, Duchenne muscular dystrophy, cardiovascular diseases, certain cancers, and viral infections like HIV and HBV.
What are the ethical considerations of CRISPR/Cas9?
Ethical concerns include germline editing, potential off-target mutations, and the risk of “designer babies.” Regulatory oversight and global ethical frameworks are essential to ensure responsible use.
How does CRISPR/Cas9 differ from traditional gene-editing techniques?
Unlike older methods like ZFNs or TALENs, CRISPR/Cas9 is simpler, faster, cost-effective, and capable of targeting multiple genes simultaneously with high precision.
Can CRISPR/Cas9 be used for cancer treatment?
Yes, CRISPR/Cas9 can modify T cells for CAR-T therapy, knock out oncogenes, or restore tumor suppressor function, improving immune response and treatment efficacy.
Conclusion
CRISPR/Cas9 is revolutionizing precision medicine by offering unprecedented accuracy, versatility, and efficiency in genome editing. Its applications span genetic disorders, cancers, viral infections, and rare diseases, enabling personalized therapies tailored to individual genetic profiles. Recent advancements in high-fidelity Cas9 variants, base and prime editing, and advanced delivery systems have significantly enhanced safety and effectiveness. As ethical frameworks, regulatory oversight, and technological innovations continue to evolve, CRISPR/Cas9 is poised to transform healthcare, offering hope for curing previously untreatable conditions and shaping the future of personalized medicine.